Chelated Iron for Plants - Types and Uses


Chelation is a process whereby metal ions are bonded to other molecules to create the resulting metal chelate. When iron is the metal involved, the result is a product known as chelated iron. They are often chelated to amino acids such as glycine or synthetic organic compounds such as EDTA and EDDHA. The purpose of doing this is to allow iron to be better absorbed by organisms and become more bioavailable. For plants, this can help them to recover when they show signs of damage such as yellow leaves or even to help their slow growth.
At thedailyECO, we look at chelated iron for plants. We discover the different types and their uses of chelated iron supplements to see if they might be useful for your outdoor or indoor plants.
What is chelated iron?
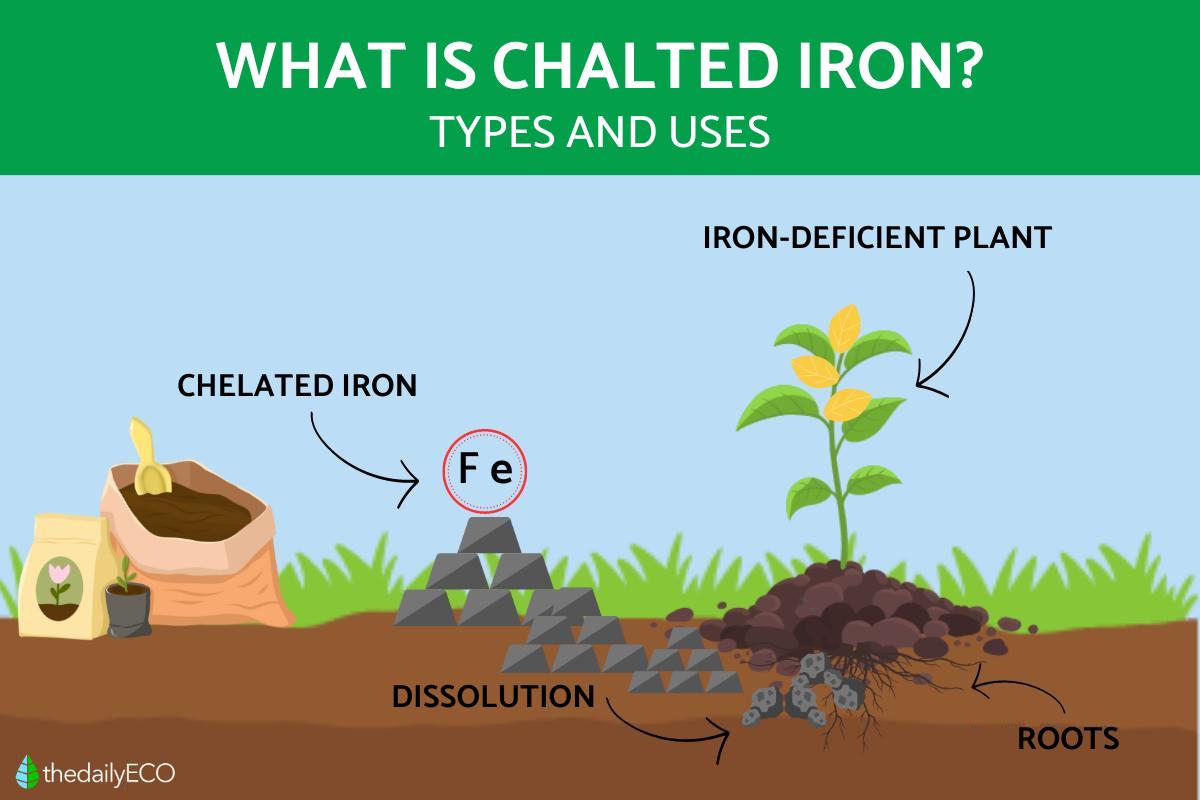
Iron chelates are a metal complex used in iron-deficient plants. They are formed by chelation, a process which can be used to bond the ions of various metals to other molecules. To do so, this process requires a chelating agent, something which will determine the type of chelate created.
Specifically for chelated iron, these agents work to stabilize the iron ions, surrounding them and binding them to the metal. What results from the bonding process is that oxidation and precipitation of iron is avoided. This increases the bioavailability of the chelated iron, meaning it can be absorbed more easily by the plant's organism. Such an increased in bioavailability has numerous benefits for the plant.
Types of chelated iron for plants
There are different types of iron chelate, which will depend on the molecules or ions used to bond the iron ions. Deciding which chelate you will want to use on your plant will depend largely on the pH of the soil. This is because some chelated iron will work best in acidic soils and others in alkaline. It is important to determine the pH of the soil before using chelated iron because using the wrong type will be of no use. The type of chelated iron you use must be suitable for the needs of the individual plant.
The two main types of chelates are:
- EDTA (ethylenediaminetetraacetic acid): is not as efficient in base or alkaline pH soils. It is used in soils with a pH level of 6.5 to 7.
- EDDHA (ethylenediaminedi(o-hydroxyphenylacetic acid)): is the most used chelated iron type because it is very efficient and the most stable. It is effective in the long term and can be used in soils with a pH level of 3 to 11, a fairly wide range that covers the spectrum of almost all soils, both acidic and alkaline.
Other, less popular types of iron chelates are:
- DPTA (diethylenetriaminepentaacetic acid): for pH of 4.5 to 7
- HEEDTA (hydroxyethylethylenediaminetriacetic acid): for pH from 4.5 to 7
- EDDCHA, EDDHMA, EDDHSA: used for pH less than 11.
On the labels of chelated iron products you will also see a percentage. This represents the amount of iron they contain. They are usually around 6% and by using them on our plants, we will have the solution to recover our plants with a deficiency of said metal.
You can purchase iron chelate with EDDHA using the link below:

How is chelated iron for plants used?
Iron chelates are used when the soil is deficient in iron or unable to make it available for use by the plant. If you do not know if your soil is iron deficient, you will be able to detect this problem since the plant will manifest certain symptoms. These symptoms are a result of the impediment to chlorophyll production due to lack of iron.
The first symptom is iron chlorosis. This occurs when the plant leaves begin to turn yellow, starting from the tip and moving to the stem. It can also manifest as yellow veins on still green leaves. If the soil is too acidic, there is a likelihood of iron chlorosis. This is because low pH does not allow iron absorption. If your plant is not growing at the expected rate, it may also be a sign that it is iron deficient.
Although yellow leaves can be due to a lack of iron, it is important to know there are other causes of this problem. Learn more with our article on why my rose leaves are turning yellow.
There are certain plants that are more prone to iron deficiency and benefit from chelated iron. Some of them are:
- Fruit trees: lemon tree, orange tree, olive tree, among others.
- Acidophilous plants: these are plants that need acidic soil such as hydrangea, rose bushes, gardenia, oak, or Japanese maple. Discover the types of hydrangea in our related guide.
Chelated iron is available as granules or in a liquid format. Both are soluble in water and can be found as fertilizer. The amount to use varies by manufacturer, so you have to pay attention to what the label indicates. As a reference, between 2 to 6 kilos of iron chelate per hectare are usually used. For indoor plants, this is about one tablespoon per liter.
Chelated iron is applied by irrigation to the soil or by foliar spray, spraying the prepared solution on the leaves with a spray bottle. In plants that have constant iron chlorosis, the dose is once a month. It is not recommended to apply more frequently because it will be counterproductive. Furthermore, its use is very common in soils that are very alkaline, where greater absorption efficiency is needed.

Difference between iron chelates and iron sulfates
Different forms of iron can be found in garden stores, the most common being sulfates and chelates. The question arises as to what the difference is between the two and whether they serve the same purpose. While they do essentially serve the same purpose, the differences between the two products are the following:
- Chelated iron has a chelating agent, but sulfates do not.
- Chelated iron is better absorbed by the roots than the sulfate.
- Sulfate is less stable to changes in pH, with the chelate being more stable.
- The chelate is purer than the sulfate.
- For all of the reasons above, iron chelate is more expensive than sulfate.
After having learned about iron chelates for plants, in this other article you can learn much more about what is iron sulfate for plants?
If you want to read similar articles to Chelated Iron for Plants - Types and Uses, we recommend you visit our Plant care and cultivation category.
- Taiz, L., & Zeiger, E. (2007). Plant physiology. Spain: Universitat Jaume I.