How Is Coal Made?

The formation of coal is one of Earth's most extraordinary natural processes, where ancient plant material undergoes a dramatic transformation over millions of years, evolving into a vital energy resource. This fossil fuel, a cornerstone of industrial development since the 18th century, remains a significant contributor to global energy production. From swampy beginnings to a solid fuel source, coal’s journey entails intricate chemical and physical changes driven by specific environmental conditions.
In this article by thedailyECO, we explore the process of coal formation, exploring the stages it undergoes, its various types, wide-ranging uses, and the environmental implications of its extraction and consumption.
What is coal?
Coal is a sedimentary rock composed primarily of carbon (65-95%), along with hydrogen, oxygen, nitrogen, sulfur, and various minerals.
While significant coal deposits formed during the Carboniferous Period (359-299 million years ago), coal formation has occurred throughout Earth's history, including the Permian, Jurassic, and Cretaceous periods.
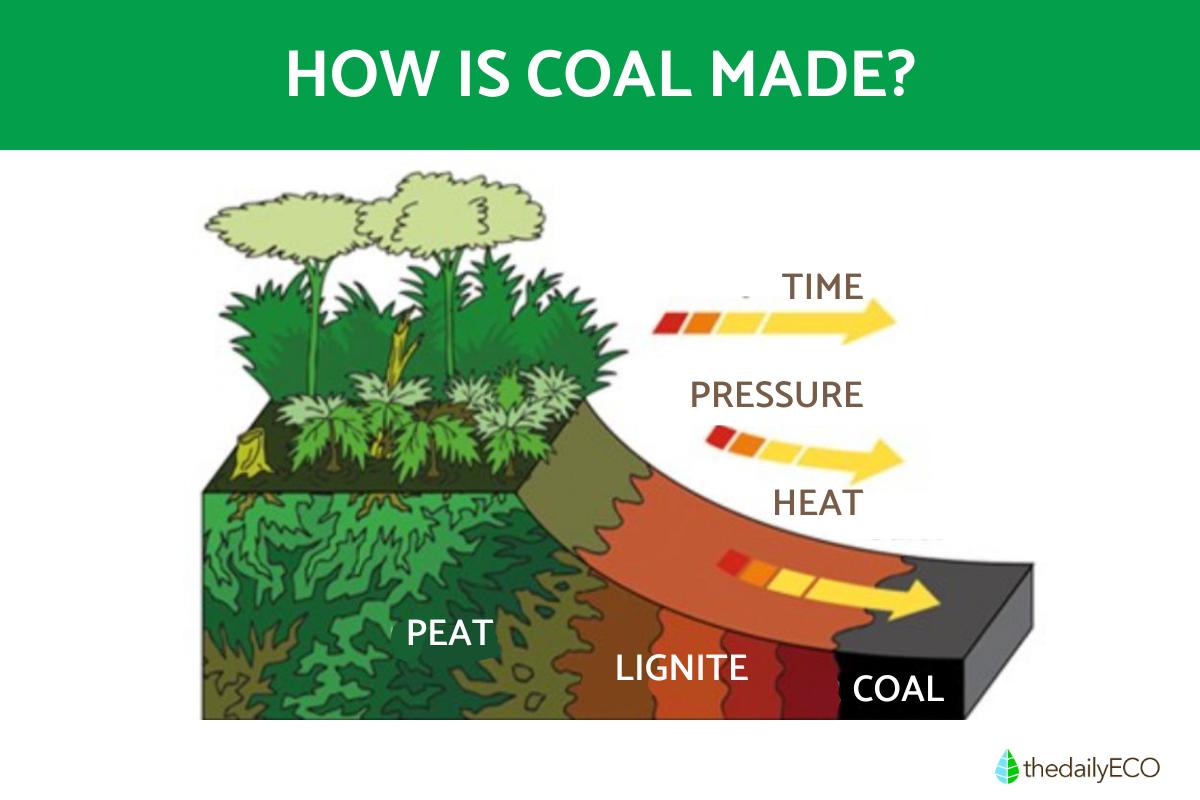
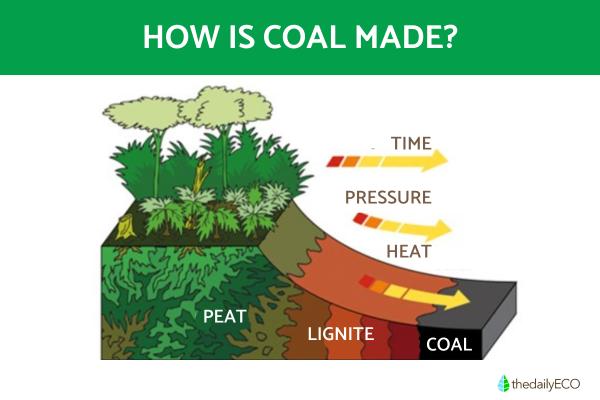
Coal originates from plant matter that accumulated in ancient swamps and wetlands. The formation process, called coalification, begins when dead plant material accumulates in oxygen-poor environments. Over millions of years, layers of sediment bury this organic matter, subjecting it to increasing pressure and temperature. These conditions, combined with the absence of oxygen, prevent normal decomposition.

Historical significance
Coal powered the Industrial Revolution, enabling unprecedented technological and economic growth. The development of steam engines and factories in 18th-century Britain marked the beginning of modern industrial society. Coal mining shaped communities, creating distinct cultural identities and labor movements.
The industry spurred technological innovations in mining, transportation, and energy production. Mining towns developed around coalfields, establishing unique social structures and economic dependencies. This heritage continues to influence modern energy policy debates and regional development, particularly in traditional mining areas transitioning to new economic models.
The rise of coal paralleled significant social changes including urbanization, labor rights movements, and environmental awareness.
Want to understand coal's place in the bigger energy picture? Discover all energy sources in our other article.
How is coal formed?
The natural formation of coal occurs through several key stages spanning millions of years:
- Plant material accumulates in wetlands and swamps, becoming submerged in water. This aquatic environment prevents normal decomposition by limiting oxygen exposure.
- Under these submerged conditions, specialized anaerobic bacteria begin breaking down the plant matter through a slow, controlled process that preserves carbon content while releasing other elements.
- Over millions of years, the organic material undergoes chemical changes as the environment becomes increasingly carbon-rich. Hydrogen and other elements are gradually lost through bacterial action and geological processes.
- Continuous sediment deposition creates overlying layers that maintain anaerobic conditions. These sediments apply increasing pressure to the organic material while raising the temperature through burial depth.
The transformation progresses through distinct stages: peat formation first, then advancing through lignite, subbituminous coal, bituminous coal, and finally anthracite. Each stage represents higher carbon concentration and energy density.
This entire process requires consistent water coverage, steady sediment accumulation, and stable geological conditions with appropriate temperature and pressure ranges.
What is the method of obtaining coal?
Coal extraction occurs through two primary methods, determined by the deposit's depth.
Open-pit mining
Open-pit mining is employed when coal deposits lie within 60 meters of the surface. This method involves systematically removing the overlying soil and rock layers, known as overburden, to expose the coal seams for direct extraction.
Open-pit mining proves more economical and generally safer than underground operations, though it creates significant surface disturbance and requires extensive land reclamation.
Underground mining
Underground mining becomes necessary when coal deposits lie too deep for surface extraction. This method involves constructing an extensive network of tunnels and shafts to access the coal seams.
Miners work through these underground passages using various techniques such as room and pillar mining, where sections of coal are left as pillars to support the roof, or longwall mining, which uses mechanical shearers to remove large panels of coal. While underground mining minimizes surface disruption, it presents greater safety challenges and requires sophisticated ventilation and structural support systems

Types of coal
Coal exists in several grades, each representing different stages of carbonization and carbon content. The transformation process, driven by time, pressure, and temperature, creates distinct types with unique properties:
- Anthracite (90-97% carbon): represents the highest quality coal, resulting from the longest formation period. This premium grade burns cleanly with minimal smoke and residue.
- Bituminous coal (70-90% carbon): features a hard texture and shiny appearance. Widely used in industrial applications and power generation.
- Subbituminous coal (35-45% carbon): serves as an intermediate grade between lignite and bituminous coal, commonly used in electricity generation.
- Lignite (25-30% carbon): has a matte black color and marks skin upon contact. Its combustion produces significant ash, reflecting its early formation stage.
- Peat: is the earliest stage, maintains high moisture content and a porous structure. Despite its low carbon content, it marks the beginning of coal formation.
- Cannel coal: is a specialized form rich in hydrogen, historically served for household lighting due to its bright, steady flame. Contains 60-70% carbon.
The fossil fuel family is bigger than you think. Learn about natural gas's role in our other article.

What are the main uses of coal?
The global coal industry centers around five major consuming nations which account for 77% of worldwide usage: China, the United States, Russia, India, and Japan. This fossil fuel serves several key industrial and domestic purposes:
- Electricity generation: coal remains a primary fuel for power plants worldwide, where it's burned to produce steam that drives electricity-generating turbines.
- Steel production: coal, particularly coking coal, is essential in steel manufacturing. It provides both fuel for the high-temperature furnaces and carbon for the steel-making process.
- Cement manufacturing: the cement industry uses coal as fuel in kilns that produce clinker, the main component of cement. The high temperatures required make coal an economical choice.
- Industrial heat applications: many industrial processes rely on coal-generated heat, including paper production, chemical manufacturing, and food processing.
- Domestic use: though declining in developed nations, coal still serves as a heating fuel in many households worldwide, particularly in developing regions.
These applications demonstrate coal's continued significance in global industry and energy production, despite growing environmental concerns and the transition toward renewable energy sources.
Environmental impact of coal
Coal's environmental footprint spans multiple ecological domains.
Its mining and use significantly affect air quality through the release of particulate matter, sulfur dioxide, nitrogen oxides, and greenhouse gases, particularly CO2.
The way coal is obtained is also problematic. On one hand, surface mining disrupts landscapes, destroys habitats, and can lead to soil erosion and water contamination. On the other hand, underground mining causes land subsidence and produces acid mine drainage, harming aquatic ecosystems.
Finally, let us not forget that coal burning produces ash and other waste products that require careful disposal to prevent groundwater contamination.
A less known fact is that the industry's water consumption, particularly in mining and power generation, strains local water resources. These impacts contribute to broader environmental challenges like climate change, biodiversity loss, and ecosystem degradation.
The journey of coal doesn't end at extraction. Find out how it influences our atmosphere in our detailed analysis of air pollutants.
If you want to read similar articles to How Is Coal Made?, we recommend you visit our Non-renewable energy category.